What Would Happen if This Cell Were Moved From Pure Water to a Seawater Solution?
Brand a Irish potato Shrink--with Saltwater
A water-moving science project from Scientific discipline Buddies
Key Concepts
Biology
Osmosis
Cells
Chemistry
Concentration
H2o transport
Introduction
Have yous always wondered how plants "drink" water from the soil? Water uptake in plants is quite complicated. A process called osmosis helps the water move from the soil into the found roots—and so into the plant's cells. In this activity you will see for yourself how you can brand water move with osmosis!
Background
Almost h2o in the ground is non pure h2o. It usually contains dissolved mineral salts. Animals and plants need these salts (which include calcium, magnesium, potassium and the sodium you might be familiar with as table salt) to grow, develop and stay healthy. Dissimilar h2o sources carry different amounts of these salts. Nature wants to balance a system that is not counterbalanced. Then if you mix h2o with two different salt concentrations, the salts don't stay separated but spread out evenly through the solution until the salt concentration is the same throughout.
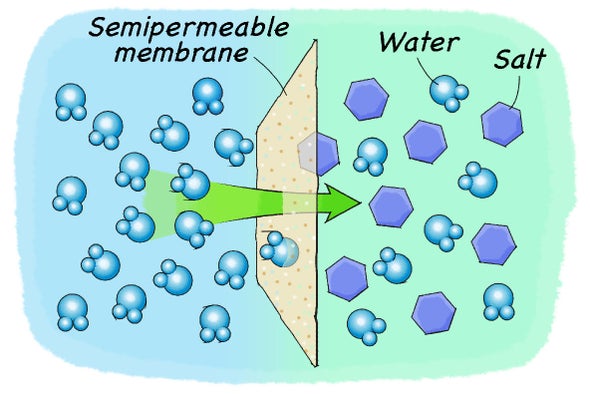
You'll notice a like reaction if you separate two salt solutions with a semipermeable membrane. A semipermeable membrane is a type of bulwark that but lets certain particles pass through while blocking others. This type of membrane usually lets water pass through just non the salts that are dissolved in the water. In this situation, because merely h2o can move through this membrane, the water will start moving from the area of lower salt concentration (which has more than water and less salt) to the surface area of college table salt concentration (which has less water and more common salt). This water motility volition simply stop in one case the table salt and water concentration on both sides of the membrane is the same.
The process of moving water beyond a semipermeable membrane is called osmosis. Plants use this process to their advantage for water uptake. They create an environment of high salt concentration in their root cells that are in contact with the soil. The prison cell walls act as a semipermeable membrane that merely allow water through. Considering the water exterior the root cells has a lower salt concentration, water starts moving into the root cells due to osmosis. The h2o entering the found fills up the cells and tin can travel to the balance of the plant. Osmosis, nevertheless, works in both directions. If you put a plant into water with a salt concentration that is higher than the concentration inside its cells, water will move out of the institute to residue out the concentration departure. Every bit a result the plant shrinks and eventually dies. You will see this effect with your own eyes in this action using potatoes and different saltwater solutions.
Materials
- Distilled water
- Measuring loving cup with milliliters (mL)
- Table salt
- Weight scale with gram measurements
- Three plastic cups or glasses
- Spoon
- At to the lowest degree three potatoes
- Apple tree corer. (Alternatively, you can take an adult help you apply a cutting board and knife.)
- Knife (and an developed helper to help you use it)
- Ruler
- Newspaper
- Pen or pencil
- Timer
- Newspaper towels
- Graphing paper (optional)
- Other vegetable(s) or fruit (optional)
Grooming
- Ready three unlike saltwater solutions. Create labels for the three cups: "0 grams," "2 grams" and "iv grams."
- To each of the cups add 100 mL of distilled h2o.
- Counterbalance out ii grams of table table salt, and add information technology to the cup that says "two grams." Then weigh out 4 grams of tabular array salt, and add it to the cup labeled "four grams." Employ a spoon to mix the solutions until all the salt is dissolved.
- Draw a tabular array in which you tin enter the starting measurements (length and diameter or width) and end measurements of each potato strip for every salt concentration (0, 2 and iv grams).
- Prepare at least three potato cores. Carefully push the corer all the way through the tater, and remove the cadre carefully so the tater piece stays intact. (Alternatively, yous can have an adult help cut the spud into strips that all have the same dimensions.) The potato pieces should be at least one-half inch thick and two inches long. (Ideally you lot volition be able to prepare nine matching cores or strips then you can test three pieces in each solution to compare the results thoroughly.)
- Employ a knife to carefully remove any tater skin from your cores, and rinse the cores quickly with h2o.
- Use a ruler to ensure each tater slice is the same size (ideally to the millimeter). Carefully utilize a knife to trim any pieces as needed.
- Measure the dimensions (length and diameter or width) of each white potato strip in millimeters, and write the information in the tabular array.
- Optionally, y'all tin also weigh each potato piece and record their weights.
Process
- Put one spud strip (or three if you made nine pieces) into each of the cups. While yous exercise that feel the potato strips with your fingers and try to flex them a trivial bit. How exercise they experience? Are they easy to bend?
- Start your timer for 30 minutes. Allow the spud strips sit down in the unlike solutions for the whole time. What do you think will happen to the strips in each of the cups?
- Later xxx minutes inspect the spud strips inside the solutions. Exercise you encounter whatever changes?
- Take the tater strip(south) out of the "0 grams" cup and place on a paper towel. While doing that experience the murphy pieces again and try to bend them slightly. How do they feel? Are they easier or more difficult to bend than before?
- Use the ruler to mensurate the exact length and bore or width (in millimeters) of each of the potato strips, and write the results in your table. What do you notice about the white potato strip measurements? Optionally you lot tin can counterbalance these pieces and record their weights.
- Next take the potato strips from the "ii grams" cup, and place them on a newspaper towel; every bit you practise this feel them. Measure out their lengths and diameters or widths. Write your results in the tabular array. Optionally you tin can weigh these pieces and tape their weights. What changed most these potato strips?
- Repeat the aforementioned steps with the potato strips in the "iv grams" cup. Write your results in the tabular array. Are your results for these like or unlike compared with the other ones?
- How did the feeling of the strips compare based on what solution they were in? Why exercise you think this is?
- Compare the results in your table. How did the length and bore or width of the white potato strips change in each cup? What virtually the weights if you lot took them? Can you explain your results?
- Extra: If you weighed each of your strips earlier and after soaking them, compare the weights. How does the mass of the potato strips modify in each solution?
- Extra: Leave the spud strips in the solutions for a longer fourth dimension period. How exercise they look if you allow them soak in the saltwater for ane hour or overnight?
- Extra: If yous have graphing newspaper, brand a graph of your results with the salt concentration on the horizontal axis and the spud strip length or diameter subsequently soaking on the vertical centrality. Describe two lines to make your graph. For the first, connect each of the information points you found. For the second, describe a horizontal line starting at the point on the vertical centrality that shows the original length of your white potato strip. Based on your graph can you lot find a salt concentration at which the murphy strip length should not change at all?
- Extra: How does the activity work with other vegetables or fruit? Try it to find out!
Observations and Results
Did your potato strips shrink and expand? At the first all the potato strips should have had the same length and should have all felt the same. When yous put them into the different solutions, withal, this starts to change. Whereas the potato strips in the "0 gram" loving cup probably got larger in size, the other potato strips probably got shorter later on leaving them in the saltwater for thirty minutes. (If you didn't encounter any pregnant changes after xxx minutes, get out the spud strips in the saltwater solutions longer.)
The shrinking and expanding of the spud strips is due to osmosis. Potatoes are made of cells, and their cell walls act equally semipermeable membranes. The 0 grams solution contains less salts and more than water than the spud cells (which have more salts and less h2o). To balance out these concentration differences, the water from the cup moves into the potato cells. The incoming water in the potato cells pushes on the cell walls and makes the cells bigger. As a issue the whole potato strip gets bigger. The reverse is the instance in the higher concentration salt solutions. If the salt concentration in the cup is higher than inside the tater cells, water moves out of the potato into the cup. This leads to shrinkage of the potato cells, which explains why the potato strips become smaller in length and diameter. Due to the shrinking of the potato cells the spud strip also becomes less rigid. If you aptitude the potato strips, you should take noticed that those that had been in the solution with the highest amount of table salt were much easier to bend than the potato strips in the water without table salt.
If you fabricated the graph y'all probably noticed that there is a salt concentration at which the potato strip neither expands nor shrinks. This should be where your information curve and your offset length line intersect. At this point the salt concentration inside the potato cells and inside the cup are the same. Because the concentrations are already balanced no water moves.
Cleanup
Discard the saltwater solutions in the sink. Throw the potato strips into the compost, and clean upwardly your workspace. You can cook with the other pieces of unused murphy.
More than to Explore
Osmosis, from Biological science Dictionary
Do Fish Drink? from McGill Academy's Office for Science and Society
Cucumber Chemistry: Moisture Capture with Desiccants, from Scientific American
Suck Information technology Up! How Water Moves Through Plants, from Scientific discipline Buddies
Stalk Activities for Kids, from Scientific discipline Buddies
Source: https://www.scientificamerican.com/article/make-a-potato-shrink-with-saltwater/
0 Response to "What Would Happen if This Cell Were Moved From Pure Water to a Seawater Solution?"
Post a Comment