What Role Did Mycorrhizae Play in the Transition of Plants to Land?
Abstruse
Mycorrhizal symbiosis between soil fungi and land plants is one of the virtually widespread and ecologically important mutualisms on globe. It has long been hypothesized that the Glomeromycotina, the mycorrhizal symbionts of the majority of plants, facilitated colonization of land by plants in the Ordovician. This view was recently challenged by the discovery of mycorrhiza-like associations with Mucoromycotina in several early on diverging lineages of land plants. Utilizing a large, species-level database of plants' mycorrhiza-like associations and a Bayesian approach to state transition dynamics we here testify that the recruitment of Mucoromycotina is the all-time supported transition from a non-mycorrhizal state. Nosotros further establish that transitions between different combinations of either or both of Mucoromycotina and Glomeromycotina occur at high rates, and institute similar promiscuity among combinations that include either or both of Glomeromycotina and Ascomycota with a nearly fixed clan with Basidiomycota. Our results portray an evolutionary scenario of evolution of mycorrhizal symbiosis with a prominent role for Mucoromycotina in the early stages of land constitute diversification.
Introduction
Land plants diverged from aquatic algae in the Neoproterozoic equally a lineage that would somewhen undergo the ecological transition to terrestrial lifeone,2. This transition – a major turning point in the history of life on earth – reshaped the global climate and the biosphere through an increment in atmospheric oxygen levels, carbon fixation, and biotic chemical weathering of rocks3,4. Terrestrial life requires plants to excerpt nutrients and wet from the substrate. Every bit roots only evolved after the transition to land5, initial plant colonization of the terrestrial surroundings was likely facilitated through interactions with symbiotic fungi where the latter provided inorganic nutrients and water to the host plant and received carbohydrates in return3,6.
Mycorrhizal symbiosis is constitute in over 90% of extant state constitute species, and all major lineages of country plants, except for mossesseven,8. Land plants form mycorrhiza-like associations with members of three different fungal phyla: Mucoromycota, Basidiomycota, and Ascomycota9,ten. The groovy majority of land plants acquaintance with arbuscular mycorrhizal fungi from the Mucoromycota subphylum Glomeromycotina, while other types of symbiotic associations, such as ectomycorrhiza, ericoid mycorrhiza, and orchid mycorrhiza, are formed by fungi of the Basidiomycota or Ascomycota9. Fossil evidence suggests that Glomeromycotina have coevolved with land plants for at least 407 Myr, as vesicles, spores, intracellular coils, and arbuscule-similar structures resembling extant symbiotic colonizations were institute in Rhynie Chert fossils of Horneophyton lignieri 11. Farther back up for ancient origin of these interactions comes from genomics, as genes involved in the germination of arbuscular mycorrhizal colinizations are homologs and were acquired in a stepwise manner, with potentiation starting as early as the last common ancestor of Charophytes and Embryophytes12,13,14.
This evidence has led to the wide credence of the view that Glomeromycotina were the ancestral mycorrhizal symbionts of land plantsxv,sixteen. The ancestral symbiosis is assumed to have been replaced in several plant lineages by other types of symbiotic associations in multiple independent shifts7,17. However, the recent discovery that many members of early on diverging lineages of land plants, including liverworts, hornworts, and early diverging vascular plants, appoint in mycorrhizal symbiosis with the Mucoromycota subphylum Mucoromycotina, challenged this hypothesis and suggests that either Mucoromycotina rather than Glomeromycotina could have facilitated terrestrialisationsixteen, or that early land plants formed dual Mucoromycotina-Glomeromycotina partnerships18,19,20,21. After this discovery, Rhynie Chert fossils where re-evaluated, revealing mycorrhiza-like colonizations resembling both Glomeromycotina and Mucoromycotina11. Moreover, mycorrhiza-germination genes from Mucoromycotina-associated liverworts recover the Glomeromycotina-associated phenotype in a transformed mutant of the angiosperm Medicago truncatula, which reveals that the genes required for symbiosis have been conserved amid liverworts that acquaintance exclusively with Mucoromycotina also as higher plants that associate exclusively with Glomeromycotina13,20.
Given that Ascomycota, Basidiomycota, Glomeromycotina, and Mucoromycotina likely diverged prior to the divergence of land plants22,23, it is possible to treat different combinations of symbiotic clan with these phyla equally categorical character states on the plant phylogeny and analyse transition dynamics between the states in a Bayesian phylogenetic comparative context. Considering the doubtfulness of the evolutionary relationships of early on Embryophytes24,25, we assessed the probability of all possible combinations of mycorrhizal associations for the almost recent mutual ancestor of land plants.
Results
We obtained a dataset of 732 species of land plants for which the mycorrhizal fungi accept been identified with molecular methods. 45 species were added to represent not-mycorrhizal lineages. We used the plant chloroplast DNA markers psbA, rbcL and rps4 to infer phylogenetic relationships between these species. Our estimates of phylogeny stand for well with the prevailing understanding of the systematics of the state plants at to the lowest degree so far as the monophyly of major groups and the relative branching order of these groups under the dissimilar rooting scenarios are concerned26,27.
Optimising the observed repertoires of mycorrhizal association as transitioning categorical states on our phylogenetic estimates resulted in a full general design of phylogenetic conservatism: major found groups acquaintance quite uniformly with major fungal groups (Fig. 1). Our ancestral state reconstructions recover strong support for the presence of mycorrhiza-similar association for the about recent common ancestor of the land plants. However, the particular state for the root was equivocal, showing comparable levels of back up for i) an association just with Mucoromycotina, two) a repertoire comprising both Mucoromycotina and Glomeromycotina; and iii) no mycorrhizal association at all (Figure S1). The relative levels of back up, and the inclusion of additionally supported root states, were influenced by different rooting scenarios (Figure S2).
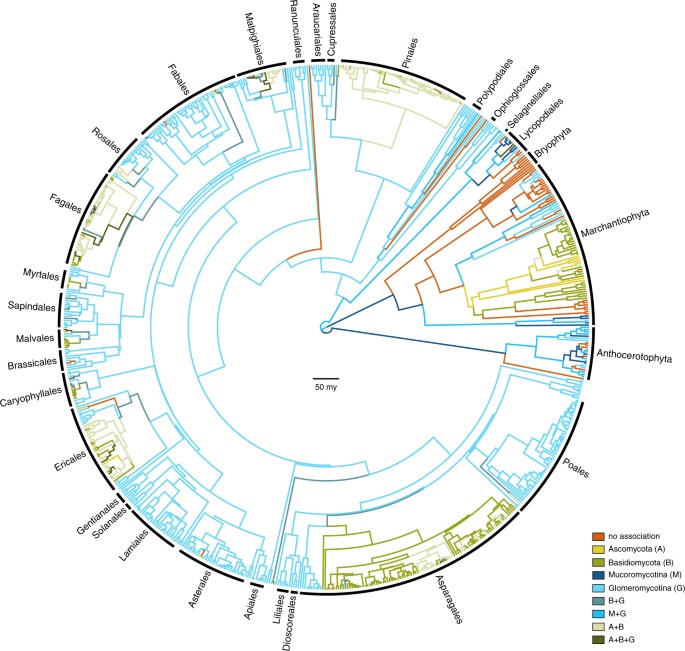
Evolution of mycorrhiza-like associations in land plants. Chronogram showing the bequeathed country reconstructions of mycorrhiza-similar associations in land plants (n = 732 species) using a phylogenetic hypothesis in which a clade consisting of liverworts and mosses are the sister grouping of all other land plant species. Branches are coloured co-ordinate to the most probable state of their ancestral nodes. Master plant lineages are marked with black labels. Branch lengths represent time in million years. Bar is fifty 1000000 years.
The design of transitions among unlike repertoires of symbiotic association suggests two chief paths along which private associations within a larger repertoire are gained and lost relatively promiscuously (Fig. 2). The offset of these paths involves Mucoromycotina and Glomeromycotina: the association with Glomeromycotina is added to, and subtracted from, the association with Mucoromycotina at relatively loftier instantaneous transition rates. The association with Mucoromycotina within a repertoire that spans both is also lost at relatively high rates, only gained at much lower rates, suggesting that the association with Glomeromycotina is relatively more facultative within this repertoire. The second path includes gains and losses of Ascomycota, and losses of Glomeromycotina (but gains less so), at high rates within repertoires in which the association with Basiodiomycota appears more obligate.
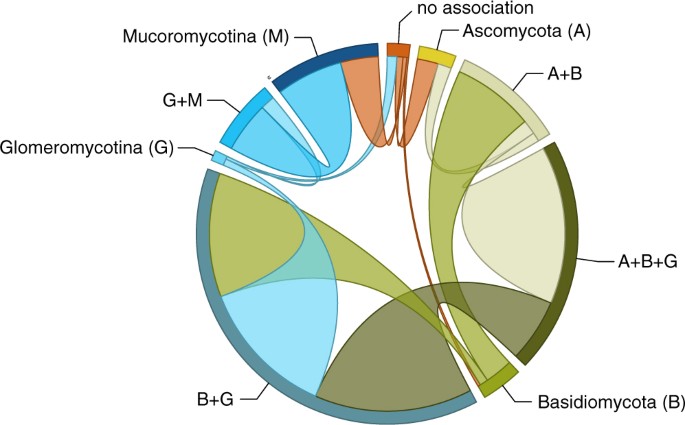
Transitions of mycorrhiza-like associations in country plant evolution. Frequency of transitions between different repertoires of mycorrhiza-similar clan equally optimised on our phylogeny (Fig. 1). The band size for each state (labelled next to the bands) represents the number of transitions from that state proportional to the total number of reconstructed transitions; and the width of the ribbons is proportional to the numbers of transitions starting from that state.
Explicit hypothesis testing to quantify which transition away from a state of no mycorrhizal association is all-time supported prefers Mucoromycotina under all four rooting scenarios: in three out of four, the Bayes Factor (BF) was larger than 10, interpreted as strong support, in the fourth scenario (hornworts sister to all other land plants) the BF was ~8.35, which is generally interpreted equally substantial support28 (Table S1). Placing the evolution of mycorrhizal associations on a temporal axis in a sliding window analysis (Fig. 3) shows Mucoromycotina and Glomeromycotina dominating early associations, while associations that include Basidiomycota and Ascomycota get more pervasive later in land plant evolution.
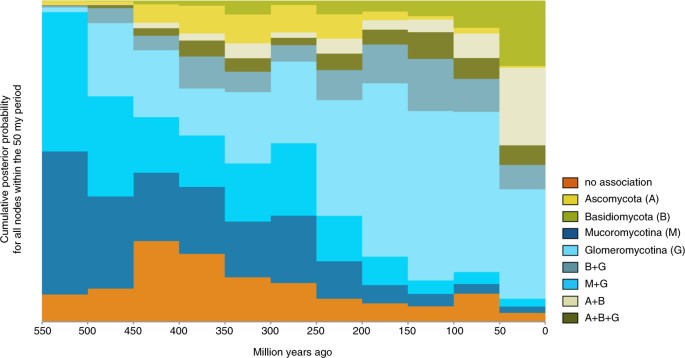
Evolution of mycorrhiza-like associations through time. The proportion of each mycorrhizal state relative to the total number of branches at that particular point in time, sampled at l million year intervals on our phylogeny with ancestral land reconstructions (Fig. 1).
Discussion
For each evaluated scenario of land plant development, our results back up the hypothesis that the most contempo common ancestor of land plants was involved in symbiotic interactions with fungi. This result is in accordance with testify from the fossil recordxi and genomics12,13,14. For the small, rootless, leafless plants with rhizoid-based absorbing systems that started colonizing the land, the brotherhood with fungi is hypothesized to take been essential in overcoming major issues of food and h2o limitation in the absence of existing soils29,30. Our analyses suggest that the fungal associates of these primeval land plants nearly probable included Mucoromycotina, and non exclusively Glomeromycotina, as commonly assumed1,31,32. An sectional association with Mucoromycotina for the root of the land plants received the highest support of all possible mycorrhizal repertoires, for all hypotheses of the relationships between the chief land found lineages. Furthermore, our hypothesis tests supported Mucoromycotina over Glomeromycotina equally the initial gain for the well-nigh recent common ancestor of the land plants. However, our reconstructions likewise advise that a repertoire comprising both Mucoromycotina and Glomeromycotina cannot be ruled out, and we find high rates for transitions in which Glomeromycotina are gained and lost in combination with Mucoromycotina (Fig. 2), suggesting a versatile scenario for the development of association with both groups. Mucoromycotina have been recorded in the rhizoids and roots of extant liverworts16, hornwortseighteen, lycophytes21, ferns21, gymnosperms33,34, and angiosperms35, only within early diverging land found lineages (except for the liverwort lineage Haplomitriopsida)16 they were generally found simultaneously with Glomeromycotina16,xviii,21. The association with both fungal lineages was likely likewise present in the Devonian fossil plant Horneophyton ligneri 11, and Field et al.nineteen speculated that the ability to acquaintance with more than i fungal partner was an aboriginal strategy that allowed the earliest land plants to occupy highly heterogeneous and dynamic environments. However, this plasticity appears to non be maintained: once association with Mucoromycotina is lost, reversals occur at a low charge per unit (Fig. 2) resulting in a predominance of strictly Glomeromycotina associations in extant plants. The scenario presented hither is contingent on our electric current understanding of the early on diversification of fungi and plants. Both Mucoromycotina and Glomeromycotina are role of the monophyletic phylum Mucoromycotax, and their difference has been estimated to predate the colonization of land by plants23. Yet, extant symbiotic species of Mucoromycotina are part of the society Endogonales10,sixteen,36 and the phylogenetic position of this group – and thus the timing of its origin relative to the emergence of state plants – remains to be investigated. Moreover, a recent molecular clock analysis estimated the living clade of land plants to have emerged in the middle Cambrian−Early on Ordovician2, which presents the possibility of an interaction between early country plants and the common ancestor of Mucoromycota23. Under this alternative scenario, symbioses formed past Mucoromycotina and Glomeromycotina result from a single evolutionary event within fungi, and, consequently, this would imply that other nutritional strategies within Mucoromycota (mostly institute pathogens and decomposers)10 represent derived states within this group. Under our current understanding of the evolution and nutritional modes of early on diverging fungi, this scenario is unlikely37.
From the prevalent association with strictly Glomeromycotina, there have been multiple independent evolutionary shifts towards Ascomycota and Basidiomycota, leading to increasingly prevalent reconstruction of these interactions over the form of establish diversification (Fig. iii). Our results advise that these transitions started with a gain of Basidiomycota, rather than Ascomycota (Fig. ii). Subsequent gains of Ascomycota and losses of Glomeromycotina occur at high rates, leading to various association repertoires that include either or both Ascomycota and Basidiomycota. These repertoires are present in several extant land plant lineages and represent the ectomycorrhizal, orchid mycorrhiza, and ericoid mycorrhizal types9,17. The ability to recruit saprotrophic lineages of wood and litter decomposable fungi from amongst Ascomycota and Basidiomycota into novel symbioses was probable instrumental for plant accommodation to diverse ecological challengesfive. For example, for Orchidaceae, the most species-rich lineage of non-arbuscular mycorrhizal plants, the transition from associations with Glomeromycotina to Ascomycota and Basidiomycota is linked to niche expansions and radiations, which in synchrony with the development of specialized pollination syndromes has promoted speciation in the largest family of plants on earth38,39. Similarly, the independent evolution of ericoid mycorrhiza in Diapensiaceae and Ericaceae, estimated to date back to the Cretaceous40,41, is a potential adaptation to nutrient poor, acidic soils31. Also, transitions to ectomycorrhiza independently evolved in various gymnosperm (e.g. Pinaceae, Gnetum, Taxus) and flowering plant lineages (e.g. Nyctaginaceae, Polygonaceae, Myrtaceae, Malvales, Malpighiales, Fabaceae, Fagales; Fig. one). Parallel to the latter, a shift towards fungi involved in the ectomycorrhizal and ericoid symbiosis has as well occurred in liverworts (Fig. 1). Although relatively few found species – generally trees and shrubs – are ectomycorrhizal, the worldwide importance of the ectomycorrhizal association is considerable, due to its say-so in temperate and boreal forests, and in tropical rainforests in Southeast Asia17. Ectomycorrhizal symbioses likely emerged in semi-arid forests dominated by conifers nether tropical to subtropical climates and diversified in angiosperms and conifer forests driven by a change to cooler climate during the Cenozoic42,43. Loss of mycorrhizal symbiosis has occurred from all single association states, more often than not at relatively depression transition rates (Fig. 3). These transitions are explained by plant adaptations to either nutrient-rich or extremely nutrient-poor soils, for which the benefits of the symbiosis do not outweigh its costs44. Notwithstanding, transition rates towards the non-mycorrhizal state may take been underestimated here, since several non-mycorrhizal flowering plant lineages (all with a recent evolutionary origin45) have not been included. A notable increment in the proportion of non-mycorrhzial lineages around 450-400 mya is acquired past the origin of mosses and the diversification of non-mycorrhizal liverworts. Similar to Maherali et al.45 we reconstructed a regain of symbiosis from a non-mycorrhizal ancestor for a few lineages. Considering it is not known whether the mycorrhizal symbiosis can be recovered subsequently loss, information technology is possible that this pathway may not occur in nature.
Our results portray an evolutionary scenario of development of mycorrhizal symbiosis with a prominent role for Mucoromycotina in the early stages of state plant diversification. In most plant lineages, Glomeromycotina, the ascendant mycorrhizal symbionts of extant land plants, subsequently replaced Mucoromycotina. Later on, several transitions from Glomeromycotina to various Ascomycota and Basidiomycota lineages have occurred, establishing novel mycorrhizal syndromes, such equally orchid, ericoid, and ectomycorrhizas. Our findings demonstrate the importance of Mucoromycotina fungi for our understanding of the early evolution of the mycorrhizal symbiosis. Nosotros still know very petty virtually the biological science of symbiotic Mucoromycotina36, but experimental evidence suggests they form mycorrhizas that are physiologically and functionally dissimilar from symbioses with Glomeromycota20. Their presence as symbiotic fungi in state plants has been overlooked until recentlyxvi,20, and it is probable that further screening of country plants will reveal that many more than plant taxa are associated with Mucoromycotina.
Methods
Data drove
To compile a dataset of plants and their symbiotic fungi, we searched the NCBI Nucleotide databank for records of Glomeromycotina (at the fourth dimension of the search 'Glomeromycota'), Mucoromycotina, Ascomycota and Basidiomycota that had annotations recording the plant host species (Figure S3). Subsequently, for each of these plant host species we conducted a GenBank search and reduced our dataset to all records with an rbcL sequence available for the plant host. For the remaining records, nosotros verified mycorrhizal status through literature study and discarded all unconfirmed records from the dataset. We then performed a literature search for plant orders that were non in the dataset as well as for early diverging lineages of land plants. Because it is hard to discriminate among symbioses formed by Glomeromycotina and Mucoromycotina by morphological observations, we only included mycorrhizal associations based on Deoxyribonucleic acid identification for these fungi. For lycopods, polypod ferns, hornworts and liverworts, species that were not constitute to harbour mycorrhiza-like associations during literature surveys were classified as non-mycorrhizal, although this could be a sampling artefact for some species. Furthermore, mosses and Nymphaea alba were included to stand for major non-mycorrhizal lineages. The final dataset covers 732 institute species distributed over 78 plant orders. The dataset includes 24 hornworts (11% of extant species multifariousness46), vii mosses (0.06%)46, 76 liverworts (0.84%)46, 518 angiosperms (0.18%)46, 73 gymnosperms (6.77%)46, 16 lycopods (1.24%)46, and 18 polypod ferns (0.17%)46. For these plants species, we found associations with 150 Ascomycota, 305 Basidiomycota, 385 Glomeromycotina, 28 Mucoromycotina and 45 non-mycorrhizal species (Table S2).
Deoxyribonucleic acid sequence data of the plants, including members of the mosses, were obtained from GenBank to reconstruct phylogenetic relationships. For liverworts, hornworts, polypod ferns, and lycopods, we added several species to the dataset to increment taxon sampling, resulting in a total of 759 species for phylogenetic analysis. For 146 species, full or fractional chloroplast genomes were bachelor, which we used to excerpt sequences for psbA, rbcL and rps4. For other species, rps4 and psbA sequences were downloaded, where possible, to supplement the rbcL dataset. Accession numbers are listed in the supplementary data (Table S3).
Phylogenetic analysis and departure dating
For each marker, we aligned the sequences with MAFFT v.747 using the FFT-NS-i Iterative refinement method, and so selected the exchange model with jModelTest 2.ane.1048,49. For each marker, 3 substitution schemes where tested on a neighbor-joining topology, including models with unequal base frequencies, rate heterogeneity, and a proportion of invariant sites. The GTR + I + γ model was selected for all partitions using the AIC. We performed divergence dating with BEAST2 v2.3.250 using four fossil calibration points and one historic period estimate from literature for the crown node of liverworts to date the phylogeny. We selected a uniform distribution for each of the calibration points using the minimum and maximum estimates for these nodes from literature (Table S4). We chose a Yule prior with a uniform birth charge per unit for the assay, a lognormal relaxed clock model, and estimated the clock rate. We applied the GTR substitution model with a Gamma category count of iv and estimated shape parameter value of i.0. The proportion of invariant sites was estimated (initial value 0.01) and the mean exchange rate fixed. We selected an exponential distribution for the prior on the mean substitution rate. To examination the effect of different phylogenetic hypotheses24,27,51 for the deep-time relationships of land plants on bequeathed land reconstruction, we reconstructed four phylogenetic hypotheses nether the following constraints: (1) hornworts sister to all other land plants, with liverworts and mosses in a monophyletic grouping ('ABasal'); (two) liverworts and mosses monophyletic and sister to the other country plants ('ATxMB'); (3) liverworts, mosses, and hornworts monophyletic and sister to the rest of the land plants ('TBasal'); and (4) liverworts sister to the balance of the land plants ('MBasal'; Figure S1). The first three hypotheses accept recently been proposed every bit the best-supported explanations using a large transcriptomic dataset27. The fourth hypothesis has been traditionally regarded every bit the most accurate representation of early land plant diversification (eastward.k. Field et al.19). During the MCMC analyses, trace files were updated every 1000 generations, and trees sampled every 10,000 generations, until the effective sample size of major traced parameters exceeded 200 (and all others exceeded 100) using a burn-in of 100 * 106 generations. We thus terminated the runs later, respectively, 374,735,000 generations for ABasal; 354,497,000 generations for ATxMB; 345,720,000 for MBasal; and 374,254,000 for TBasal. We so constructed the maximum clade credibility tree using Tree Annotator v2.two.ane.
Comparative analysis and hypothesis tests
In our analysis nosotros assume that the 4 major fungal groups of which members participate in symbiotic associations were already in existence prior to the diversification of land plants23. Therefore, we treat each distinct repertoire of associations that land plants course with members of these groups equally a discrete state whose evolutionary transition dynamics we modelled subsequent to two additional assumptions. First, because there are qualitative differences between the types of symbiotic associations that are formed with some of the different fungal groups (eastward.yard. intracellular versus ectomycorrhizal association), nosotros assumed that the evolutionary adaptations required to enable such associations are not gained (or lost) instantaneously. Hence, we disallowed state shifts that implied multiple, simultaneous gains and losses such that, for instance, a modify from a country representing a repertoire confined to Glomeromycotina to 1 confined to Mucoromycotina has to pass through an intermediate state where the repertoire is broadened to include both groups. Second, because the corresponding adaptations that enable different types of mycorrhiza-like association are probable subject to evolutionary trade-offs such that repertoires of associations cannot expand infinitely we express whatever intermediate states to those nosotros observe in nature. For example, simultaneous association with both Glomeromycotina and Mucoromycotina does occur in our dataset of extant taxa, but complete generalism that includes all fungal groups in a unmarried repertoire does non, which is why we immune the onetime, just not the latter, as possible ancestral states.
A user-friendly side outcome of these assumptions was that this limited the number of free parameters in the state transition (Q) matrix, which otherwise would accept undergone a combinatorial explosion had we included all possible permutations in the repertoires of mycorrhiza-like association equally distinct states, which would have impeded convergence in our analyses. To mitigate such proliferation of potentially unneeded, gratuitous parameters further, nosotros performed our analyses using Reversible-Bound MCMC, as implemented in BayesTraits's 'multistate' analysis fashion. Nosotros ran each of our analyses in triplicate for 106 generations, as initial experimentation had demonstrated reasonable convergence in our information under these settings. In cases where we required estimates of marginal likelihoods, i.e. for hypothesis testing by Bayes gene analysis, we approximated these using a stepping stone sampler that we ran for 100 stones, with 200,000 iterations per stone.
Using this approach, we reconstructed the ancestral states for the four unlike rootings of our phylogeny. Yet, although such analyses result in estimates for the posterior distribution of states at whatsoever given node (such as the root), they exercise not necessarily provide the false certainty on which to base a single, unambiguous scenario for the order in which symbiotic associations are acquired, particularly not when multiple states are reconstructed with similarly big posterior probabilities at deep nodes (as was the case). Given the number of fungal groups and the differences and similarities amongst these with respect to the types of mycorrhizal associations they participate in, nosotros expected there to exist singled-out paths along which repertoires of clan accept evolved. Interrogation and visualisation of the Q matrix showed that, broadly, two such paths announced to exist: one where diverse permutations of association with Glomeromycotina and Mucoromycotina are gained and lost, and another that traverses Ascomycota, Basidiomycota in addition to Glomeromycotina. However, which of these paths was taken first was not yet evident.
Nosotros therefore synthetic explicit hypothesis tests to distinguish betwixt various plausible scenarios. To practise so, in addition to the assumptions affecting the Q matrix outlined higher up, nosotros further constrained our analyses to require the absenteeism of any mycorrhizal clan on the root node, and and so tested which initial proceeds was best supported by the information. To quantify this, we estimated the marginal likelihood of the model where the root is constrained to take no clan simply without any additional constraints on the order in which subsequent associations are acquired (beyond the general assumptions already discussed), and compared this with models where, respectively, each of the initial gains of a single fungal group is disallowed. The logic here is that disallowing the initial shift that best fits the information volition result in the marginal likelihood that differs most significantly from the less-constrained model.
Lastly, to place the expansion of repertoires of symbiotic association on a temporal centrality, we placed the bequeathed state reconstructions for the scenario where the root node has no mycorrhizal clan in bins of 50 Myr to visualise these in a states-through-time plot (Fig. 3). All data and scripts are available (https://doi.org/ten.5281/zenodo.1037586).
Data availability
The datasets generated during and/or analysed during the current study are available in the GitHub repository, https://doi.org/10.5281/zenodo.1037586.
References
-
Delwiche, C. F. & Cooper, E. D. The evolutionary origin of a terrestrial flora. Curr. Biol. 25, R899–R910 (2015).
-
Morris, J. L. et al. The timescale of early country constitute development. Proc. Natl. Acad. Sci. Us early view, https://doi.org/10.1073/pnas.1719588115 (2018).
-
Selosse, Thou.-A., Strullu-Derrien, C., Martin, F. M., Kamoun, Southward. & Kenrick, P. Plants, fungi and oomycetes: a 400-million yr affair that shapes the biosphere. New Phytol. 206, 501–506 (2015).
-
Lenton, T. Thou., Crouch, 1000., Johnson, M., Pires, N. & Dolan, L. First plants cooled the Ordovician. Nat. Geosci. 5, 86–89 (2012).
-
Brundrett, K. C. Coevolution of roots and mycorrhizas of land plants. New Phytol. 154, 275–304 (2002).
-
Pirozynski, Thou. A. & Malloch, D. W. The origin of country plants: A matter of mycotrophism. BioSystems 6, 153–164 (1975).
-
Wang, B. & Qiu, Y. L. Phylogenetic distribution and development of mycorrhizas in land plants. Mycorrhiza xvi, 299–363 (2006).
-
Pressel, S., Bidartondo, 1000. I., Ligrone, R. & Duckett, J. G. Fungal symbioses in bryophytes: New insights in the Xx Get-go Century. Natural History 253, 238–253 (2010).
-
van der Heijden, Grand. G. A., Martin, F. Thousand., Selosse, One thousand.-A. & Sanders, I. R. Mycorrhizal ecology and evolution: the past, the present, and the future. New Phytol. 205, 1406–1423 (2015).
-
Spatafora, J. W. et al. A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia 108, 1028–1046 (2016).
-
Strullu-Derrien, C. et al. Fungal associations in Horneophyton ligneri from the Rhynie Chert (c. 407 million yr sometime) closely resemble those in extant lower land plants: Novel insights into ancestral plant-mucus symbioses. New Phytol. 203, 964–979 (2014).
-
Karandashov, V., Nagy, R., Wegmüller, S., Amrhein, N. & Bucher, One thousand. Evolutionary conservation of a phosphate transporter in the arbuscular mycorrhizal symbiosis. Proc. Natl. Acad. Sci. USA 101, 6285–xc (2004).
-
Wang, B. et al. Presence of three mycorrhizal genes in the common ancestor of land plants suggests a key role of mycorrhizas in the colonization of land by plants. New Phytol. 186, 514–525 (2010).
-
Delaux, P.-Yard. et al. Algal ancestor of land plants was preadapted for symbiosis. Proc. Natl. Acad. Sci. 112, 13390–13395 (2015).
-
Ligrone, R. et al. Glomeromycotean associations in liverworts: A molecular, cellular, and taxonomic assay. Am. J. Bot. 94, 1756–1777 (2007).
-
Bidartondo, M. I. et al. The dawn of symbiosis between plants and fungi. Biol. Lett. 7, 574–577 (2011).
-
Brundrett, Thou. C. & Tedersoo, L. Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytol. early view https://doi.org/10.1111/nph.14976 (2018).
-
Desirò, A., Duckett, J. One thousand., Pressel, S., Villarreal, J. C. & Bidartondo, M. I. Fungal symbioses in hornworts: a chequered history. Proc. R. Soc. London B Biol. Sci. 280, 20130207 (2013).
-
Field, K. J., Pressel, S., Duckett, J. G., Rimington, West. R. & Bidartondo, One thousand. I. Symbiotic options for the conquest of country. Trends Ecol. Evol. 30, 477–486 (2015a).
-
Field, One thousand. J. et al. First evidence of mutualism between ancient plant lineages (Haplomitriopsida liverworts) and Mucoromycotina fungi and its response to simulated Palaeozoic changes in atmospheric CO2. New Phytol. 205, 743–756 (2015).
-
Rimington, West., Pressel, South., Duckett, J. & Bidartondo, 1000. I. Fungal associations of basal vascular plants: reopening a closed volume? New Phytol. 205, 1394–1398 (2015).
-
Rubinstein, C. 5., Gerrienne, P., de la Puente, Chiliad. S., Astini, R. A. & Steemans, P. Early Middle Ordovician evidence for state plants in Argentina (eastern Gondwana). New Phytol. 188, 365–369 (2010).
-
Chang, Y. et al. Phylogenomic analyses point that early on fungi evolved digesting jail cell walls of algal ancestors of land plants. Genome Biol. Evol. 7, 1590–1601 (2015).
-
Cox, C. J., Li, B., Foster, P. G., Embley, T. M. & Civan, P. Alien Phylogenies for Early Country Plants are Caused past Composition Biases amidst Synonymous Substitutions. Syst. Biol. 63, 272–279 (2014).
-
Wickett, N. J. et al. Phylotranscriptomic analysis of the origin and early on diversification of land plants. Proc. Natl. Acad. Sci. 111, E4859–E4868 (2014).
-
Ruhfel, B. R., Gitzendanner, M. A., Soltis, P. S., Soltis, D. E. & Burleigh, J. G. From algae to angiosperms-inferring the phylogeny of green plants (Viridiplantae) from 360 plastid genomes. BMC Evol. Biol. xiv, 23 (2014).
-
Puttick, Chiliad. Due north. et al. The interrelationships of land plants and the nature of the bequeathed embryophyte. Current Biol. 28, 733–745 (2018).
-
Kass, R. E. & Raftery, A. Due east. Bayes factors. J. Am. Stat. Assoc. 90, 773–795 (1995).
-
Kenrick, P. & Crane, P. R. The origin and early evolution of plants on land. Nature 389, 33–39 (1997).
-
Kenrick, P. & Strullu-Derrien, C. The origin and early evolution of roots. Plant Physiol. 166, 570–580 (2014).
-
Parniske, M. Arbuscular mycorrhiza: the mother of institute root endosymbioses. Nat. Rev. Micro. 6, 763–775 (2008).
-
Smith, South. E. & Read, D. J. Mycorrhizal symbiosis, 3rd edn. London, UK: Academic Press (2008)
-
Tedersoo, L. & Smith, M. Eastward. Lineages of ectomycorrhizal fungi revisited: foraging strategies and novel lineages revealed by sequences from belowground. Fungal Biol. Rev. 27, 83–99 (2013).
-
Yamamoto, K., Endo, N., Degawa, Y., Fukuda, M. & Yamada, A. First detection of Endogone ectomycorrhizas in natural oak forests. Mycorrhiza 27, 295–301 (2017).
-
Orchard, S. et al. Fine endophytes (Glomus tenue) are related to Mucoromycotina, not Glomeromycota. New Phytol. 213, 481–486 (2017).
-
Desirò, A. et al. Multigene phylogeny of Endogonales, an early diverging lineage of fungi associated with plants. IMA Mucus 8, 245–257 (2017).
-
Berbie, M. L., James, T. & Strullu-Derrien, C. Early diverging Fungi: variety and impact at the dawn of terrestrial life. Annu. Rev. Microbiol. 71, 41–60 (2017).
-
Ogura-Tsujita, Y., Gebauer, Thou., Hashimoto, T., Umata, H. & Yukawa, T. Prove for novel and specialized mycorrhizal parasitism: the orchid Gastrodia confusa gains carbon from saprotrophic Mycena. Proc. Biol. Sci. 276, 761–767 (2009).
-
Yukawa, T., Ogura-Tsujita, Y., Shefferson, R., Yokoyama, J. & Ogura-Tsujita, Y. Mycorrhizal multifariousness in Apostasia (Orchidaceae) indicates the origin and evolution of orchid mycorrhiza. Amer. J. Bot. 96, 1997–2009 (2009).
-
Cullings, Grand. Unmarried phylogenetic origin of ericoid mycorrhizae within the Ericaceae. Tin. J. Bot. 74, 1896–1909 (1996).
-
Schwery, O. et al. Equally quondam as the mountains: the radiation of the Ericaceae. New Phytol. 207, 355–367 (2015).
-
Matheny, P. B. et al. Out of the Palaeotropics? Historical biogeography and diversification of the cosmopolitan ectomycorrhizal mushroom family Inocybaceae. J. Biogeograph. 36, 577–792 (2009).
-
Looney, B. P., Ryberg, M., Hampe, F., Sánchez-García, M. & Matheny, P. B. Into and out of the tropics: global diversification patterns in a hyperdiverse clade of ectomycorrhizal fungi. Mol. Ecol. 25, 630–647 (2016).
-
Lambers, H. & Teste, F. P. Interactions betwixt arbuscular mycorrhizal and non-mycorrhizal plants: do not-mycorrhizal species at both extremes of nutrient availability play the same game? Plant Cell Environm. 36, 1911–1915 (2013).
-
Maherali, H., Oberle, B., Stevens, P. F., Cornwell, W. Yard. & McGlinn, D. J. Mutualism persistence and abandonment during the evolution of the mycorrhizal symbiosis. Am. Nat. 188, E113–E125 (2016).
-
Christenhusz, M. J. M. & Byng, J. West. The number of known plant species in the world and its annual increase. Phytotaxa 261, 201–217 (2016).
-
Katoh, Thou. & Standley, D. Thou. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. thirty, 772–780 (2013).
-
Guindon, South. & Gascuel, O. A simple, fast and accurate method to estimate large phylogenies by maximum-likelihood. Syst Biol 52, 696–704 (2003).
-
Darriba, D., Taboada, Thou. Fifty., Doallo, R. & Posada, D. jModelTest 2: more than models, new heuristics and parallel calculating. Nature Methods 9, 772 (2012).
-
Bouckaert, R. et al. BEAST 2: a software platform for Bayesian evolutionary analysis. PLoS Comput Biol ten, e1003537 (2014).
-
Clarke, J. T., Warnock, R. C. Chiliad. & Donoghue, P. C. J. Establishing a time-scale for plant evolution. New Phytol. 192, 266–301 (2011).
Acknowledgements
We thank Andrew Meade for help with BayesTraits, and Francis Martin and two anonymous reviewers for valuable comments on a previous version of the manuscript.
Author data
Affiliations
Contributions
Five.S.F.T.M. initiated and supervised the project. F.A.A.F. compiled the data with input from J.Due north.; F.A.A.F. and R.A.V. performed the analyses. All authors wrote the manuscript.
Respective author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional data
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary textile
Rights and permissions
Open Access This article is licensed nether a Creative Commons Attribution iv.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you lot give appropriate credit to the original writer(southward) and the source, provide a link to the Creative Commons license, and betoken if changes were fabricated. The images or other 3rd party material in this article are included in the article's Creative Commons license, unless indicated otherwise in a credit line to the material. If cloth is not included in the article'south Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, yous will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and Permissions
Near this commodity
Cite this article
Feijen, F.A.A., Vos, R.A., Nuytinck, J. et al. Evolutionary dynamics of mycorrhizal symbiosis in land plant diversification. Sci Rep 8, 10698 (2018). https://doi.org/10.1038/s41598-018-28920-x
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/x.1038/s41598-018-28920-x
Further reading
Comments
By submitting a comment you hold to bide by our Terms and Community Guidelines. If you find something abusive or that does non comply with our terms or guidelines please flag information technology as inappropriate.
Source: https://www.nature.com/articles/s41598-018-28920-x
0 Response to "What Role Did Mycorrhizae Play in the Transition of Plants to Land?"
Post a Comment